Scientists are investigating the capability of gold specks (yellow spots) for conveying medicines into the system.(Image credit: Veronika Sapozhnikova, Konstantin Sokolov, Rebecca Richards-Kortum/M.D. Anderson Cancer Center and Rice University via NIH/Flickr)ShareShare by:
- Copy link
- X
Share this article 0Join the conversationFollow usAdd us as a preferred source on GoogleNewsletterSubscribe to our newsletter
When the earliest cells materialized on our planet roughly 3.8 billion years in the past, viruses were already there awaiting them. Since then, viruses have been conceiving methods to taint cells, and cells have been counteracting by advancing strategies to halt these contaminations. This developmental interplay ultimately prompted the rise of your defense apparatus.
A vital trait of your defense mechanism is to differentiate “own” from “alien” so it can abolish and clear away extraneous matter from your organism. While this immune reaction shelters you against viruses, it also carries weight for the degree to which external substances like drugs function.
I am a specialist exploring approaches to enhance the efficacy of medicines, involving methods to direct them to the ailment location within the physique prior to being eliminated or ruined. An approach to achieve this is to confine medicines within nanoparticles — materials minute enough to be imbibed by cells. Although these substances still initiate an immunological response to purge them from the body, scientists like myself have determined that this reaction could truly be employed to heighten the potency of cancer therapy.
You may like
-
COVID-19 mRNA vaccines can trigger the immune system to recognize and kill cancer, research finds
-
An experimental mRNA treatment counters immune cell aging in mice
-

One molecule could usher revolutionary medicines for cancer, diabetes and genetic disease — but the US is turning its back on it
The immune system and drug delivery
Aside from spotting contagions, your defense apparatus also reacts to tissue deterioration. You may discern this response as inflammation — for example, flushing and puffiness — when drugs are injected into your body via a syringe.
Ordinarily this inflammatory reaction is negligible. Nevertheless, the chance of a prolonged response escalates when drugs are given gradually across a protracted duration, for example, during chemotherapy infusions that could consume an hour or more. For this intention, certain patients are administered anti-inflammatory drugs before infusion to mitigate the potential for a negative immune response during the procedure.
The freshest advancements in channeling medicines into the organism encompass employing nanoparticles. These substances — which can be constructed from lipids, proteins, gold or other components — possess the advantage of being extremely minute: The breadth of a typical nanoparticle is roughly 10-thousandths of a millimeter. Their diminutive dimension permits diseased cells to effortlessly absorb them. Thus, when nanoparticles encompass medicines, they can function as a drug transport framework.
The Mighty Power of Nanomaterials: Crash Course Engineering #23 – YouTube

Watch On
Despite being exceptionally minute, nanoparticles are capable of holding a significant quantity of drug constituents, empowering them to convey a potent collection of therapy straight into a cell. They can also transport drugs composed of DNA and RNA. The most recognized instance of this technology is the COVID-19 inoculation, which utilizes nanoparticles fashioned from modified fat molecules to transmit mRNA that instructs the defense apparatus to safeguard itself against COVID-19 contamination.
Your inherent immune system also identifies nanoparticles as extraneous trespassers when they are channeled into your body. Consequently, a few patients undergo an initial inflammatory reaction when the organism attempts to assault the nanoparticle.
But envision if this reaction could genuinely be harnessed to refine treatment?
Exploiting the innate immune response
For the prior 3 decades, my laboratory at the University of Colorado has been scrutinizing the manner in which nanoparticles convey drugs. Increasingly recently, we’ve concentrated on comprehending the manner in which the innate immune system counters an injection of nanoparticles. While this immunological reaction is typically regarded as a hindrance, we aspired to probe whether it could improve therapy.
You may like
-
COVID-19 mRNA vaccines can trigger the immune system to recognize and kill cancer, research finds
-
An experimental mRNA treatment counters immune cell aging in mice
-

One molecule could usher revolutionary medicines for cancer, diabetes and genetic disease — but the US is turning its back on it
In a 2022 investigation on the impact of nanoparticles on the immune response in rodents, we observed that the innate immune response sparked by a preliminary quantity of nanoparticles transporting a medicine will furthermore lessen the consequence of a subsequent quantity if it is injected shortly subsequently — generally within days. It achieves this by eradicating the medicine from the organism more swiftly. This response mirrors the manner in which a primary viral infection would initiate a short-term protective response against a succeeding infection from another virus.
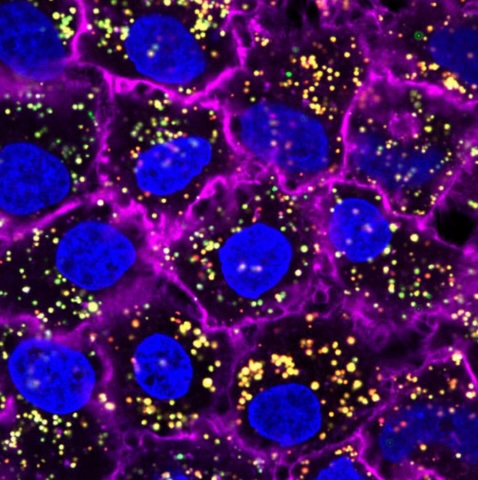
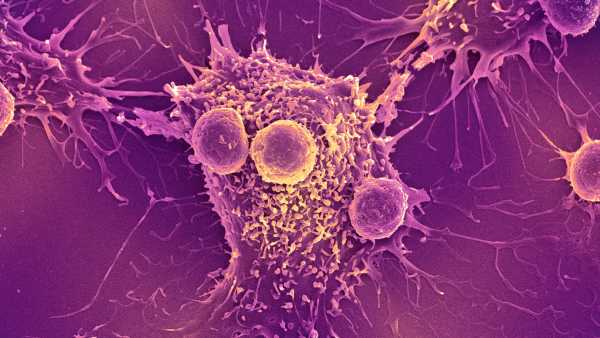
Nanoparticles — the yellow spots — can be crafted to target cancer cells, which are blue.
A vital aspect of this defensive impact involves the production of a protein termed interferon lambda. This molecule “interferes” with the infection progression by hindering viruses from acquiring admittance to diverse tissues within the organism. Researchers have formerly evaluated this protein as a prospective antiviral drug for treating COVID-19.
Similarly, the interferon lambda generated in response to the preliminary quantity of nanoparticles curtails the capability of the subsequent quantity to convey the medicine to healthy tissues within the body. However, it did not influence the nanoparticle’s aptitude to access tumors, conceivably owing to the fact that tumors can impair the immune response.
In standard cancer therapy, chemotherapy drugs are utilized to eradicate the tumor. Owing to the fact that these drugs are likewise toxic to healthy cells, patients frequently experience side effects for example hair thinning, gastrointestinal complications and skin rashes. Employing nanoparticles to channel cancer therapy could aid in diminishing these side effects, and uniting them with interferon lambda could permit the nanoparticle-encapsulated drug to remain in the body sufficiently long to manifest its complete impacts.
RELATED STORIES
—100-year-old heart drug made from foxglove may help ‘dissolve’ clumps of spreading cancer cells
—Combo of cancer therapy drugs increases mice lifespan by 30% — but anti-aging benefits in humans remain unknown
—’Universal’ cancer vaccine heading to human trials could be useful for ‘all forms of cancer’
Our group is probing whether directly injecting interferon lambda ahead of chemotherapy with nanoparticles could assist in restricting the quantity of drug that concludes in healthy tissues while elevating their density in tumors. In an initial trial of this strategy in rodents with colon cancer, all rodents that were administered interferon lambda experienced boosted survival duration and lessened weight decline. An enhanced understanding of how this consequence unfolds could enable researchers to eventually assess this approach to cancer therapy in human patients.
Scientists have a protracted path to traverse in formulating nanoparticles that are as competent as viruses at permeating into cells. But our aspiration is that exploiting an immune response that advanced approximately a billion years previously to avert viral contaminations could contribute to lessening the toxic side effects from therapy while refining its efficacy.
This edited article is republished from The Conversation under a Creative Commons license. Read the original article.

Tom AnchordoquyProfessor of Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus
Tom Anchordoquy is a professor at the University of Colorado School of Pharmacy, where he has been on the faculty since 1998. His research focuses on the design of nanomedicines, including lipid-DNA complexes and exosome-based drug delivery systems, as well as strategies that harness nanoparticle-induced immune responses to improve cancer treatment. His lab also conducts formulation studies using small molecules to treat a range of diseases.
Show More Comments
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
LogoutRead more
COVID-19 mRNA vaccines can trigger the immune system to recognize and kill cancer, research finds
An experimental mRNA treatment counters immune cell aging in mice

One molecule could usher revolutionary medicines for cancer, diabetes and genetic disease — but the US is turning its back on it
It matters what time of day you get cancer treatment, study suggests
‘Chemo brain’ may stem from damage to the brain’s drainage system
