“`html
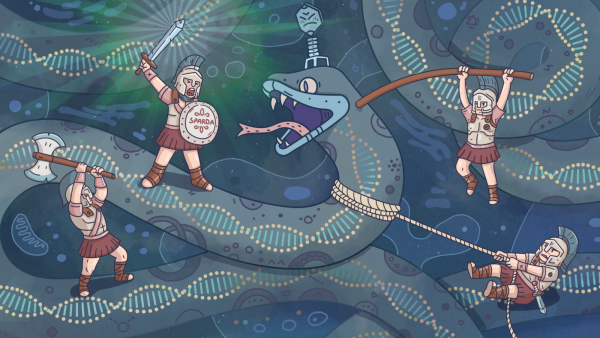
An artistic interpretation featuring SPARDA as it defends a bacterial cell from a virus invasion.(Image credit: Justinas Griciunas)ShareShare by:
- Copy link
- X
Share this article 0Engage in the discussionTrack usAdd us to your favored sources on GoogleMailing ListSign up for our newsletter
CRISPR initiated an age of discovery within genetic studies; yet, in the natural world, similar mechanisms with unexplored capacity are abundant. Recently, researchers have achieved substantial progress in deciphering the operational principles of a mysterious system termed SPARDA.
CRISPR methodologies have furnished scientists with tools to manipulate genetic material with unprecedented ease. While most recognized for its employment in gene modulation, CRISPR is, in actuality, a repurposed bacterial immunity apparatus, adapted for usage within human contexts.
You may like
-

Metal compounds identified as potential new antibiotics, thanks to robots doing ‘click chemistry’
-
Newfound antibiotic shows ‘100 times’ more potency against drug-resistant bacteria than its predecessor
-

One molecule could usher revolutionary medicines for cancer, diabetes and genetic disease — but the US is turning its back on it
Molecular argonautes
Mindaugas Zaremba, the co-author of the study and a biochemist operating from Vilnius University in Lithuania, conveyed to Live Science that preceding this recent endeavor, inquiries into SPARDA frameworks had been constrained. They had ascertained that constituent proteins forming the system execute a sacrificial mechanism for cell defense, safeguarding a wider collective of bacteria from extraneous DNA, encompassing free-form DNA entities termed plasmids, along with viruses designated as phages.
“SPARDA infrastructures have been shown to insulate bacteria from plasmids and phages through disintegration of DNA sourced from both compromised cells and invading entities, culminating in the demise of the host cell while simultaneously forestalling further propagation of the infectious process amidst the bacterial populace,” Zaremba elucidated.
The underlying molecular functionalities of SPARDA persisted in obscurity, instigating Zaremba and his team to utilize the AI-driven protein assessment instrument known as AlphaFold, along with a compilation of additional diagnostic protocols, intending to delve into the arrangement of SPARDA. AlphaFold leverages machine learning to forecast the three-dimensional configuration of proteins contingent on the sequence of their underlying building elements.
The architectural framework of SPARDA is built upon argonaute proteins, so named due to their resemblance to argonaut octopuses (Argonauta). Initially, such proteins were recognized within plant organisms, wherein seedlings exhibited attenuated foliage attributed to mutations impacting these proteins, reminding scientists of an octopus’s tentacles. These argonaute proteins are phylogenetically maintained and exist within cells throughout the three biological domains.

An argonaut octopus, source of the name for argonaute proteins.
Zaremba’s investigation analyzed SPARDA systems, chosen at random, from a pair of bacterial species. The initial one, Xanthobacter autotrophicus, flourishes as a soil-based microbe, eluding sunlight while synthesizing sustenance from local nitrogen derivatives. The subsequent species, Enhydrobacter aerosaccus, initially surfaced within Michigan’s Wintergreen Lake, possessing integrated flotation devices facilitating its mobility throughout watery environments.
Zaremba’s collective excised the SPARDA architectures from these bacterial specimens, relocating them into E. coli, an established model organism, for closer inspection. Molecular analysis revealed that each argonaute protein encompassed an essential “activating region.” They designated this region as the beta-relay, owing to its resemblance to electrical relay circuits that manipulate machinery via toggling between “on” and “off” configurations.
Upon detection of external hazards by the SPARDA structures, these switches underwent conformational shifts. This novel morphology empowered proteins to create complexes alongside supplementary activated argonaute proteins. Subsequently, the proteins array themselves in a manner akin to soldiers undergoing a parade, establishing lengthy, spiraling formations. These chains then cleave any surrounding DNA they happen upon, culminating in an extreme reaction that indiscriminately eradicates both host and intruder alike. This thwarts further proliferation of the contagion to other cells.
You may like
-

Metal compounds identified as potential new antibiotics, thanks to robots doing ‘click chemistry’
-
Newfound antibiotic shows ‘100 times’ more potency against drug-resistant bacteria than its predecessor
-

One molecule could usher revolutionary medicines for cancer, diabetes and genetic disease — but the US is turning its back on it
Zaremba’s group then used AlphaFold to analyze similar bacterial proteins for the presence of beta-relays. The identical switches recurred persistently, insinuating that relays constitute a widespread attribute associated with this class of proteins.
SPARDA in diagnostics
While SPARDA is indispensable for bacterial defense mechanisms, Zaremba’s cohort posit that this system might also render assistance to humans.
Activation of SPARDA signifies an ultimate measure for bacterial cells, employed exclusively when an infection is unambiguously detected. Consequently, the framework integrates an exceptionally precise recognition system for identifying exogenous DNA meriting self-inflicted eradication.
Zaremba proposed that researchers could potentially repurpose the system for diagnostic applications. In such a framework, the beta-relay could be refined to be activated solely upon detection of a specific genetic sequence of interest—for instance, reacting exclusively upon encountering genetic elements pertaining to influenza or SARS-CoV-2 viruses. This principle underlies currently employed CRISPR-based diagnostic resources.
RELATED STORIES
—US infant receives unprecedented personalized CRISPR therapy targeting genetic disorder
—Innovative CRISPR alternative can ‘install’ complete genes, clearing path for therapies addressing numerous genetic ailments
—A novel CRISPR system arrests gene activity, instead of irreversibly shutting them down
Nevertheless, current CRISPR diagnostic techniques are constrained in functionality, only acknowledging targets adjacent to particular DNA segments, termed PAM sequences. Analogous to prongs on a plug, these sequences require a matching socket to activate the system. Thus, the selection of an adequate CRISPR protein that corresponds to a specific target constitutes a critical step.
“We are already aware that SPARDA systems function irrespective of a PAM sequence,” Zaremba stated. The absence of such a requirement would allow it to act as a universal adapter, enhancing the versatility of future DNA diagnostics, which would ultimately improve the ability of tests to detect a spectrum of pathogens.
CRISPR research earned a Nobel Prize and revolutionized the landscape of science. Though SPARDA research remains in its initial stages, its core mechanics propose that the designs seen in microscopic organisms could provide insights into the most complex scientific challenges.

RJ MackenzieLive Science Contributor
RJ Mackenzie is an award-nominated science and health journalist. He has degrees in neuroscience from the University of Edinburgh and the University of Cambridge. He became a writer after deciding that the best way of contributing to science would be from behind a keyboard rather than a lab bench. He has reported on everything from brain-interface technology to shape-shifting materials science, and from the rise of predatory conferencing to the importance of newborn-screening programs. He is a former staff writer of Technology Networks.
Show More Comments
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
LogoutRead more

Metal compounds identified as potential new antibiotics, thanks to robots doing ‘click chemistry’
Newfound antibiotic shows ‘100 times’ more potency against drug-resistant bacteria than its predecessor

One molecule could usher revolutionary medicines for cancer, diabetes and genetic disease — but the US is turning its back on it

DNA from ancient viral infections helps embryos develop, mouse study reveals

‘Biological time capsules’: How DNA from cave dirt is revealing clues about early humans and Neanderthals
