“`html

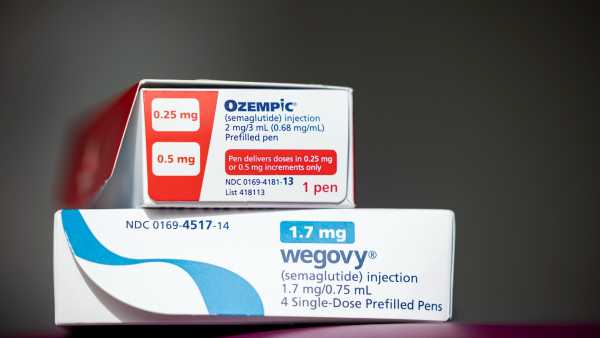
The recently introduced oral form of Wegovy performs just as effectively as the injectable version, according to studies.(Image credit: Novo Nordisk)ShareShare by:
- Copy link
- X
Share this article 0Join the conversationFollow usAdd us as a preferred source on GoogleNewsletterSubscribe to our newsletter
The Food and Drug Administration (FDA) has given the green light to an oral variant of the well-known weight-loss medication Wegovy, which is now accessible via various pharmacies and online healthcare providers throughout the nation.
Since 2021, the drug has been obtainable via prescription in the form of a once-per-week shot. Similar to the injectable, the updated Wegovy tablet comprises semaglutide, the identical functioning constituent as Ozempic. It functions by imitating a bodily hormone — glucagon-like peptide-1 (GLP-1) — which aids in the regulation of hunger and glucose levels.
Both modalities of Wegovy hold approval for assisting individuals dealing with obesity — alongside overweight adults who possess linked medical issues, like elevated hypertension — in reducing weight and keeping it off, when paired with a diet featuring fewer calories and amplified physical exercise. Nevertheless, the injectable version exclusively has the authorization for children aged 12 and above suffering from obesity; the oral rendition isn’t yet sanctioned for that demographic.
You may like
-
Study links GLP-1 use to some pregnancy risks — but the research has key caveats
-
A fentanyl vaccine enters human trials in 2026 — here’s how it works
-

Has America’s obesity rate plateaued?
Listed below is what you should understand concerning the updated Wegovy tablet, plus which individuals stand to reap the greatest benefits from it.
Do semaglutide pills work as well as injections?
The crucial distinction between the Wegovy tablet and the injection pertains to the manner in which the medication reaches the circulatory system.
Whenever semaglutide gets injected below the skin’s surface, it’s immediately drawn into the bloodstream. Conversely, when administered as a tablet, the drug is compelled to transit via the belly and intestines, in which a portion of its potent constituent is disassembled via digestive enzymes prior to its capacity to get soaked up through the intestinal lining and enter the blood.
As a consequence, “solely a small piece [of the tablet] makes its way into the bloodstream,” clarified Dr. Chika Anekwe, obesity medicine clinical director of the Massachusetts General Hospital Weight Center and an instructor in medicine at Harvard Medical School. This represents an anticipated dissimilarity between injected versus oral medicaments, in broader terms.
Even though the oral form subjects the digestive pathway to a greater quantity of semaglutide when compared against the injection, its localized impacts upon the gut remain insignificant, Anekwe stated. That’s on account of the reality that the “primary effects still mandate absorption along with systemic signaling,” consequently they solely activate upon the drug’s arrival into the bloodstream, she expressed.
To compensate for that digestive operation, the oral version of Wegovy gets taken at a notably elevated dosage compared to the injectable version. The uppermost dosage for the tablet registers at 25 milligrams, in contrast to 2.4 milligrams for the once-weekly injection, Anekwe communicated to Live Science via email.
The tablet additionally accompanies explicit guidelines regarding the opportune moment for ingestion, Anekwe revealed. It ought to be ingested on an empty belly during the morning alongside a minimal quantity of water, and individuals must postpone ingesting food or fluids or taking supplementary medications for a duration of at least 30 minutes, per its drug label stipulations. This guarantees the medication’s appropriate absorption at the intended dose. The injectable form lacks these restrictions; it’s permissible to administer it at any point during the day without any mandated fasting.
You may like
-
A fentanyl vaccine enters human trials in 2026 — here’s how it works
-

Has America’s obesity rate plateaued?
-
New drug could prevent diabetes complications not fixed with blood sugar control, study hints
Dr. Priya Jaisinghani, an endocrinologist and obesity medicine specialist at NYU Langone Health, addressed the efficacy of the two formulations during an Endocrine Society symposium regarding GLP-1 tablets back in December 2025.
Thus far, “no long-standing, head-to-head trial has been conducted” to juxtapose the once-daily 25-mg tablet with the once-weekly 2.4-mg injection among adults coping with obesity, Jaisinghani stated. Nonetheless, findings sourced from clinical trials assessing the medications independently imply that the two variants yield quite comparable weight diminution upon adhering to directions.
Within the STEP-1 trial, a probe into 2.4-mg injectable semaglutide, participants underwent a mean reduction of about 14.4% of their overall weight. Inside the OASIS 4 trial, an assessment of the 25-mg tablet, participants experienced a mean depletion of roughly 13.9% of their overall weight. Jaisinghani communicated that these outcomes signify that the tablet and the injection “presented analogous effectiveness concerning weight regulation.”

This signifies a 1.5-mg starter dosage pertaining to the Wegovy tablet. The tablets encompass an augmented dosage of the active ingredient in comparison to the injections, due to the tablets’ mandate to traverse the digestive apparatus. Is the Wegovy pill as safe as the injection?
The trials additionally insinuate that Wegovy tablets and injectables generate comparable adverse effects.
“Oral as well as injectable Wegovy induce exceptionally akin, predominantly gastrointestinal adverse reactions,” Anekwe stated. These span from queasiness and looseness of the bowels to emesis and sluggishness, for instance.
Roughly three-quarters of Wegovy adopters undergo mild-to-moderate gastrointestinal symptoms, Anekwe articulated. Though concerning both configurations of the medication, a fraction of individuals — approximately 7% to 10% — suspend utilization of the medicament in light of these consequences, according to trial details.
(Some actual data implies that a more substantial proportion of individuals cease utilization of GLP-1s within a year from inception, albeit this may stem from a convergence of elements, encompassing both adverse reactions and medication expenses.)
Who might benefit more from the pill or the injection?
Individuals contemplating Wegovy may settle upon the tablet or injection stemming from varying rationales. As an illustration, “the tablet could constitute a superior alternative for an individual harboring reluctance regarding the utilization of injections,” Anekwe communicated.
The tablet version might additionally hold amplified allure for persons devoid of dependable access to refrigeration, such as frequent voyagers, she expressed. This stems from the tablet’s capacity for storage at ambient temperature, diverging from the injection’s stipulation for refrigeration. Broadly speaking, the Wegovy injectables ought to be sustained between 36 and 46 degrees Fahrenheit (2 to 8 degrees Celsius). Further, when the need arises, the pens are amenable to storage at 46 to 86 F (8 to 30 C) for a span of up to 28 days, contingent upon their caps’ intactness.
RELATED STORIES
—Ozempic-style drugs tied to more than 60 health benefits and risks in biggest study-of-its-kind
—Study links GLP-1 use to some pregnancy risks
—Ozempic-style drugs treat type 1 diabetes, not only type 2, study finds
On that note, the rigid daily regimen entailing tablet consumption on an empty stomach accompanied by restrictions pertaining to food timing might pose adherence hurdles for particular individuals. Those persons could potentially favor the straightforwardness offered by a once-weekly injection.
Financial implications and insurance safeguarding might similarly sway an individual’s preference concerning the version of choice. The tablet could represent a superior option for an individual devoid of insurance coverage in light of its diminished out-of-pocket financial burden, Anekwe stated.
Transcending lifestyle determinants and financial implications, there subsists an additional crucial variance dictating eligibility concerning each version: Diverging from the injectable configuration of Wegovy, which garners sanction for adolescents aged 12 and beyond experiencing obesity, the Wegovy tablet presently attains authorization solely for adults.
Disclaimer
This article is for informational purposes only and is not meant to offer medical advice.
TOPICSnews explainers

Clarissa BrincatLive Science Contributor
Clarissa Brincat functions as a freelance writer specializing in health and medical research. Subsequent to securing an MSc in chemistry, she came to the realization that she harbored a preference for articulating science rather than practicing it. She acquired proficiencies concerning the editing of scientific papers through a tenure as a chemistry copyeditor, preceding her transition into a medical writer role situated within a healthcare enterprise. Authorship targeting doctors and experts harbors inherent merits, albeit Clarissa aspired to engage a more extensive audience, which naturally directed her toward freelance health and science writing. Her body of work has additionally materialized within Medscape, HealthCentral, in conjunction with Medical News Today.
Show More Comments
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
LogoutRead more
A fentanyl vaccine enters human trials in 2026 — here’s how it works

Has America’s obesity rate plateaued?
New drug could prevent diabetes complications not fixed with blood sugar control, study hints
A ‘functional cure’ for HIV may be in reach, early trials suggest
