(Image courtesy of Knowable Magazine, CC BY-ND)
Back in 2008, neurovirologist Renee Douville discovered something strange in the brains of people who died from amyotrophic lateral sclerosis (ALS): viral proteins.
But these people were not infected with any known virus.
Instead, ancient genes that originally belonged to viruses and were still lurking in the chromosomes of these patients awakened and began producing viral proteins.
You may like
-
Scientists have discovered how viruses hidden in our DNA control our genes
-
Vaccines show promise in fight against dementia
-
New pocket-sized UAS model 'breathes and flows like human tissue'
Our genomes are littered with fragments of long-forgotten viruses, descendants of viral infections that often arose millions of years ago. Most of these once-foreign fragments of DNA are a type of retrotransposon; they make up more than 40% of the human genome.
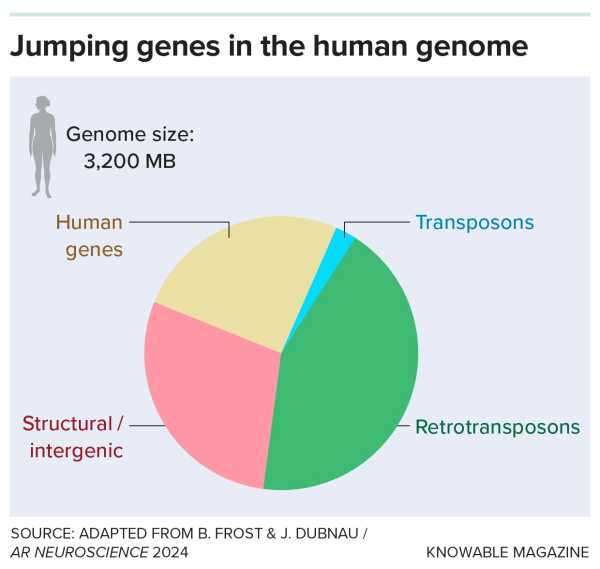
Our genomes are riddled with DNA from ancient viral infections known as “jumping genes.” Most are retrotransposons, which copy themselves via RNA intermediates; a smaller number are DNA transposons, which copy and paste fragments.
Our genomes are riddled with DNA from ancient viral infections known as “jumping genes.” Most are retrotransposons, which copy themselves via RNA intermediates; a smaller number are DNA transposons, which copy and paste fragments.
Many retrotransposons appear to be harmless in most cases. But Douville and other researchers are exploring the possibility that some newly awakened retrotransposons could cause serious harm: They could destroy nerve cells and cause inflammation, and they may underlie some cases of Alzheimer's disease and amyotrophic lateral sclerosis (ALS).
The theory linking retrotransposons to neurodegenerative diseases—conditions in which nerve cells degrade or die—is still evolving; even its proponents, while optimistic, are cautious. “It’s not a consensus view yet,” says Josh Dubnau, a neuroscientist at the Renaissance School of Medicine at Stony Brook University in New York. Retrotransposons cannot explain all cases of neurodegeneration.
Still, evidence is accumulating that they may underlie some cases. Now, after more than a decade of exploring the possibility in human brain tissue, fruit flies, and mice, the researchers are putting their ideas to the ultimate test: clinical trials in people with ALS, Alzheimer’s, and related conditions. These trials, using antiretroviral drugs from the HIV pharmacopoeia, have yielded preliminary but promising results.
Meanwhile, scientists are still studying how a viral reactivation turns into a full-blown disease, a process that may be marked by what Dubnau and others call a “retrotransposon storm.”
Genes that jump
A retrotransposon is a kind of “jumping gene.” These pieces of DNA can (or once could) move around the genome, being copied or deleted from one place and then inserted into a new one. Retrotransposons are copyists and inserters.
Many retrotransposons are old satellites: some of them predate the evolution of Homo sapiens or even the split between plants and animals, Dubnau says. Their predecessors may have alternately been stitched into and out of the host chromosome, he suggests.
Some retrotransposons, after all this time, retain the ability to move around human DNA. To do this, they copy themselves using the enzyme reverse transcriptase, which is also used by some viruses, such as HIV, to copy RNA sequences into DNA. Once copied, the remaining viruses can occupy new positions on chromosomes.
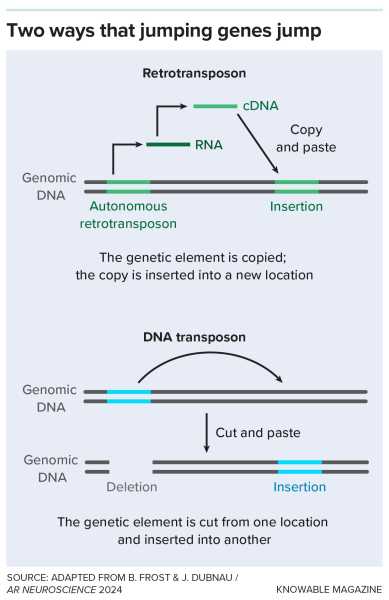
Jumping genes use different mechanisms to move around the genome.
If you're terrified of a genome studded with retroviral genes, some of which can move around the genome, don't worry, says Douville, now at the University of Manitoba in Winnipeg. Remarkably, some retrotransposons have taken on useful jobs, helping the body with tasks such as maintaining stem cells, developing embryos, and developing the nervous system.
Many retrotransposons are dormant or damaged, and the cell has ways of allowing them to (mostly) remain undetected. One way is to hide them in stretches of DNA that are so tightly coiled that the molecular machines needed to copy genes can’t get to them.
Essentially, the camera pushes them into a closet and slams the door.
But evidence is accumulating that as we age, the cabinet door may creak, allowing retrotransposons to escape. What exactly they do is still unknown. Some scientists believe it is not so much that they move around and mutate DNA, but that their virus-like RNA and proteins can disrupt normal cellular activity.
“I think the toxicity of transposon activation is really because they produce all these factors that are perceived by the cell as a virus,” says Bess Frost, a neuroscientist at Brown University in Providence, R.I. The cell responds, quite logically, with the protective inflammation that is typically associated with neurodegeneration.
Retrotransposons also appear to associate with pathological proteins traditionally associated with neurodegeneration, damaging or killing nerve cells and perhaps even triggering the disease in the first place.
Establishing communication with the UAS
Scientists have long suspected a link between viruses and amyotrophic lateral sclerosis (ALS), a disease that causes degeneration of the motor neurons that control movement. But when the connection was finally discovered, it wasn’t quite what they thought.
In the early 2000s, scientists reported that some people with ALS had a viral enzyme called reverse transcriptase in their blood and, rarely, in their cerebrospinal fluid. In some people, levels of reverse transcriptase were as high as those in people with HIV.
But at the time, Dubnau says, “nobody could find the virus.”
Finally, Douville and his colleagues found traces of one of these residual viruses, a variant of the retrotransposon HERV-K, in the brains of some people who had died from ALS. From there, scientists began to build evidence linking jumping genes to ALS in people, lab animals, and cell cultures. In 2017, a group of scientists reported that multiple jumping genes were activated in the brains of some people with ALS.
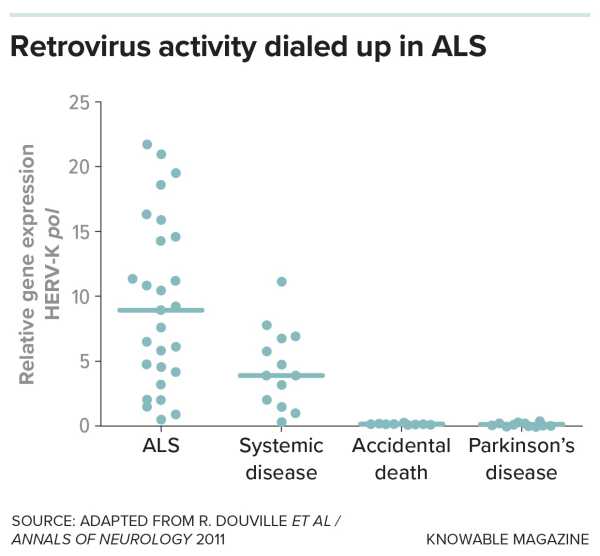
In one of the first studies linking retroviruses and neurodegeneration, researchers looked for evidence that genes for the retrovirus HERV-K were upregulated in the brain tissue of people who died from ALS compared with those who died from other causes. The activity of a HERV-K gene called pol, which is involved in copying the retroviral genome, was elevated in many ALS samples.
Douville's colleagues also documented the damage HERV-K caused: When they introduced the gene from the retrotransposon into mice, the animals' nerve cell processes shriveled and they developed ALS-like symptoms.
When scientists zeroed in on what might activate HERV-K, they found a familiar protein. It was called TDP-43, and it had already been linked to ALS. But even before that, it had been found to be involved in how cells respond to the retrovirus HIV.
In the 1990s, scientists discovered that TDP-43 acts in the cell nucleus to prevent HIV genes from activating. It also regulates the activity of human genes. However, in neurons from people with ALS or a related disorder, frontotemporal dementia (FTD), TDP-43 leaves the nucleus and forms abnormal clumps in the cytoplasm. These clumps are associated with a number of neurodegenerative diseases and can spread from cell to cell. When TDP-43 leaves the nucleus, it also creates a disruption in gene regulation, disrupting the activity levels of many genes.
Damage to TDP-43 is enough to cause neurodegeneration, but research suggests that its loss of nuclear function can also activate retrotransposons. A study of brain cells from people who died of ALS or FTD found that when TDP-43 left the nucleus, the tightly coiled DNA around certain retrotransposons began to loosen and unwind. The researchers also found that in cultured cells, the loss of TDP-43 freed certain retrotransposons from their confines. The closet door was now ajar, meaning retrotransposons were free to jump out.
Meanwhile, Dubnau and his colleagues were studying data on TDP-43 and the genes it controls in rats, mice, and humans. They found that TDP-43 could naturally attach to the RNA of various jumping genes, suggesting that normal TPD-43 could continue to block them even if they had managed to copy themselves into the RNA. This interaction was disrupted in people with frontotemporal dementia and in rodents with abnormally high or low levels of TDP-43—suggesting that TDP-43 could no longer control the jumping genes.
The Dubnau team also turned to fruit flies. Both old age and the human TDP-43 gene caused retrotransposons in the fly brains to escape from the chromosomal “closet,” prompting brain cells to kill their neighbors and triggering neurodegeneration, the team reported in a series of papers published between 2013 and 2023. What’s more, activation of certain retrotransposons also caused TDP-43 to clump together outside the nucleus, creating a vicious cycle in which TDP-43 and the retrotransposons reinforce each other’s abnormal behavior. “After a certain point, everything just picks up speed,” Dubnau says.
Taking all of this evidence together, Dubnau proposes a possible mechanism for ALS: Normally, TDP-43 in the nucleus is involved in silencing retrotransposons. But if aging or some other disorder causes TDP-43 to be deactivated, these previously suppressed retrotransposons become activated, producing virus-like RNAs and proteins. Although retrotransposons can cause disease on their own by occupying new sites in DNA or causing inflammation, they also act on TDP-43. They cause more TDP-43 to leave the nucleus and accumulate in the cytoplasm, causing further neurodegeneration that spreads to neighboring cells.
This is not the cause of all ALS, as it is a complex disease with many possible triggers. However, in a 2019 study of postmortem brain samples, Dubnau and colleagues found that about one in five people with ALS had high levels of retrotransposon activation and TDP-43 dysfunction.
The Tau and Alzheimer's Link
As the ALS story unfolded, other scientists were exploring the connection between retrotransposons and another toxic protein that causes neurodegeneration: tau, a protein that curls into unruly tangles in the brain cells of people with Alzheimer’s. It affects retrotransposons, Frost says, because, like TDP-43, it plays a role in keeping them active when they’re not.
This maintenance is the result of tau’s association with the cell’s internal skeleton. This skeleton is physically linked to the structure of the nucleus, which in turn anchors the tightly coiled DNA that suppresses retrotransposons. When tau is broken down, it alters the structure of the cell’s basic skeleton, making it more rigid. Frost and his colleagues found that this structural defect extends all the way to the nuclear skeleton and chromosomes, much like how pulling on the threads on one side of a net can change the shape of the other side.
In 2014, Frost reported that this structural effect could unlock tightly coiled sections of chromosomes in fruit flies, damaging their neurons. By 2018, she had shown that tau problems release jumping genes in flies.
“They were actually jumping,” she says, moving from their original chromosomal sites to other sites in the fly’s brain cells. And these jumping genes contributed to the death of nerve cells.
“They actually jumped.”
Bess Frost
Frost and his colleagues also studied mammals—mice—and reported in 2022 that retrotransposons were also activated in mice with tau dysfunction.
Meanwhile, Frost and others examined brain cells from people who died from tau-related diseases, such as Alzheimer's, and also found activated retrotransposons.
According to a 2022 paper by another team, this awakening of retrotransposons appears to occur early in the disease. In blood samples from people on the path to developing Alzheimer’s, the copying of retrotransposon genes into RNA increased dramatically, causing a “retrotransposon storm” just before symptoms became severe enough to be called Alzheimer’s.
HIV treatment tactics
A growing body of evidence suggests that reactivation of previously “silent” retrotransposons, whether through dysfunctional tau or TDP-43, can wreak havoc. A potential treatment immediately comes to mind: Because these retrotransposons are so much like viruses, scientists believe antiviral drugs could help.
Fortunately, doctors now have drugs that block retroviruses: millions of people take antiretroviral drugs to keep HIV under control or to prevent it from gaining a foothold in their cells.
Indeed, numerous studies over several years have examined drugs in the HIV arsenal that block the enzyme reverse transcriptase. In cells, flies, and mice, these drugs reduced retrotransposon activity and neurodegeneration.
These drugs are well-studied and generally safe, and are already being tested in neurodegenerative diseases. For example, researchers tested the safety of a 24-week course of antiretroviral therapy in 40 patients with ALS. Most participants not only completed the study safely, but their blood levels of HERV-K decreased and they appeared to have a delay in the progression of ALS symptoms, the researchers reported in 2019.
Frost recently published the results of a small study in which 12 people with early-stage Alzheimer's disease took a reverse transcriptase inhibitor for 24 weeks. Her main goal was to determine the safety of the treatment, and it was safe. The researchers also observed a reduction in signs of inflammation in the participants' cerebrospinal fluid.
Both Dubnau and Frost serve on the scientific advisory board of Transposon Therapeutics, which tested its proprietary reverse transcriptase inhibitor in 42 patients with ALS and/or frontotemporal degeneration (FTD). The company says the drug was well-tolerated by patients and showed signs of reduced neurodegeneration and inflammation, as well as delayed the inevitable worsening of symptoms. The company is planning a larger study; it also plans to test its drug in patients with ALS, Alzheimer’s disease, and a related tau-deficiency disorder called progressive supranuclear palsy.
Neither Frost nor Dubnau, who recently co-summed up the field for the Annual Review of Neuroscience, believe that antiretroviral drugs alone are the answer to transposon-induced Alzheimer’s disease (ALS). As Douville notes, these drugs were designed to target only specific enzymes—they won’t target other retrotransposon genes, RNA, or proteins that might also trigger nerve-damaging inflammation.
Meanwhile, scientists are looking beyond ALS and Alzheimer's disease, as evidence mounts that retrotransposons may contribute to other neurodegenerative and inflammatory diseases, such as Parkinson's disease and multiple sclerosis.
“It's really picking up speed,” Frost says.
This article originally appeared in Knowable Magazine, a nonprofit publication dedicated to making scientific knowledge accessible to all. Subscribe to the Knowable Magazine newsletter.

Amber DanceScience Writer
Amber Dance is an award-winning freelance science writer based in Southern California. She is a contributor to Knowable Magazine, serves as the New Horizons in Science Briefings program director at the Council for the Advancement of Science Journalism's annual Sciencewriters Conference, and teaches Science Writing I at UCLA.
You must verify your public display name before commenting.
Please log out and log back in. You will then be prompted to enter a display name.
Exit Read more
Scientists have discovered how viruses hidden in our DNA control our genes
Vaccines show promise in fight against dementia
New pocket-sized UAS model 'breathes and flows like human tissue'
'These decisions were completely reckless': Cutting funding for mRNA vaccines will make America more vulnerable to pandemics
How the battle between viruses and bacteria could help us defeat superbugs
Scientists claim that humans may have untapped 'superpowers' in genes linked to hibernation. Latest news in genetics

Scientists discover 'spirals' in DNA that form under pressure
Special protection could help human eggs stay fresh as the body ages

8 genomic hotspots linked to ME/CFS in largest study of its kind
Scientists claim humans may have untapped 'superpowers' in hibernation-related genes
Scientists have discovered how viruses hidden in our DNA control our genes

The best-ever map of the human genome reveals 'hidden' regions of DNA. Latest news
Remnants of ancient viruses make up 40% of our genome. They can cause brain degeneration.

2,200-year-old 'intricate and graceful' Celtic warrior amulet reveals sophisticated metalworking in Iron Age

New research suggests that a catastrophic collision with a neighboring planet may be the reason for life on Earth.
This week's science news: World's first pig-to-human lung transplant and successful SpaceX Starship test flight

'I Would Never Let a Robot Carry My Baby': 'Pregnancy Robots' Poll Divides Live Science Readers

Scientists have discovered that the geology that supports the Himalayas is not what we thought. LATEST ARTICLES

1Are there any countries where there are no mosquitoes?
Live Science is part of Future US Inc., an international media group and leading digital publisher. Visit our corporate website.
- About Us
- Contact Future experts
- Terms and Conditions
- Privacy Policy
- Cookie Policy
- Accessibility Statement
- Advertise with us
- Web Notifications
- Career
- Editorial Standards
- How to present history to us
© Future US, Inc. Full 7th Floor, 130 West 42nd Street, New York, NY 10036.
var dfp_config = { “site_platform”: “vanilla”, “keywords”: “type-news-daily,type-crosspost,exclude-from-syndication,serversidehawk,videoarticle,van-enable-adviser-
Sourse: www.livescience.com





