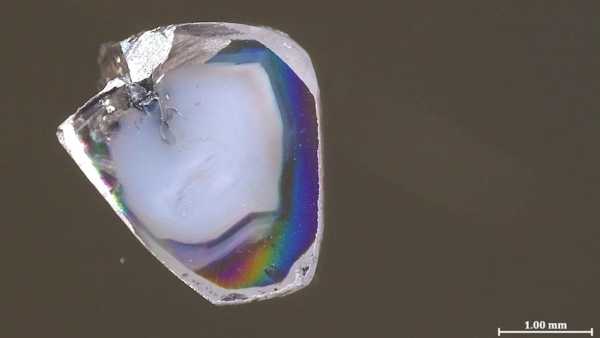
A diamond from deep within the Earth. (Photo: Yael Kempe and Yakov Weiss)
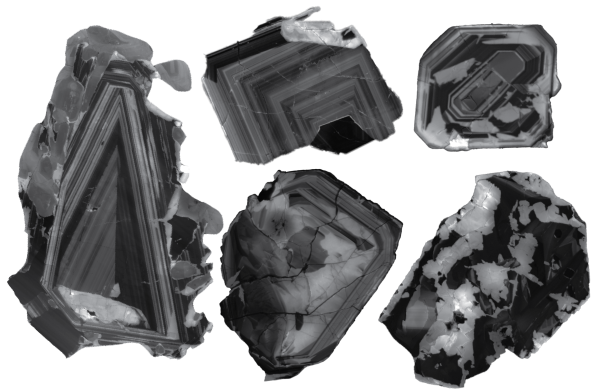
A pair of diamonds formed hundreds of kilometers deep in Earth's plastic mantle contain inclusions of materials formed under completely opposite chemical conditions—a combination so unusual that researchers deemed their coexistence “practically impossible.” The presence of these substances offers insight into the chemical processes in the mantle and the reactions that lead to diamond formation.
Two diamond samples were found in a South African mine. Like many other gemstones, they contain so-called inclusions—tiny particles of surrounding rock trapped during the diamond's formation. Most jewelers dislike these inclusions, but for scientists, they represent an intriguing source of information. This is especially true for diamonds formed deep in the inaccessible mantle, as they transport these inclusions virtually intact to the surface—this is the only way these minerals can travel hundreds of kilometers without changing their original state deep within the mantle.
Each of the two new diamond samples contains inclusions of carbonate minerals rich in oxygen atoms (known as oxidized) and oxygen-poor nickel alloys (known as reduced). Just as an acid and base react instantly to form water and salt, oxidized carbonate minerals and reduced metals don't coexist for long. Typically, inclusions in diamonds contain only one of these, so their presence puzzled Yaakov Weiss, a senior lecturer in the Department of Earth Sciences at the Hebrew University of Jerusalem, and his colleagues so much that, he says, they initially set the samples aside for a year, failing to find them.
You may like
-

We are only just beginning to learn what the Earth's inner core is really made of.
-
A huge source of the rare earth metal niobium was brought to the surface when the supercontinent broke apart.
-

Bizarre liquid 'fireworks' occur when scientists try to mix immiscible liquids.
But after reanalyzing the diamonds, the researchers discovered that the inclusions represent a snapshot of the reaction that led to the formation of these sparkling stones, confirming for the first time that diamonds can form through the interaction of carbonate minerals and reduced metals in the mantle. The new samples mark the first time that scientists have been able to observe the midpoint of this reaction in a natural diamond.
“They're essentially two ends of the [oxidation] spectrum,” says Weiss, senior author of a new study describing the find and published Monday in the journal Nature Geoscience.
This discovery has implications for understanding what lies at the mysterious center of the mantle. As you move deeper into the Earth, away from the surface, rocks and minerals become increasingly reduced, and oxygen molecules become increasingly scarce, but there is virtually no direct evidence of this change in the mantle.
Theoretical calculations gave the researchers an idea of how the planet transitions from an oxidized to a reduced state with depth. “We knew about this reduction from some empirical data obtained from real samples at depths of perhaps as deep as 200 kilometers,” says Maya Kopylova, a professor in the Department of Earth, Ocean, and Atmospheric Sciences at the University of British Columbia, who was not involved in the new study but wrote an accompanying editorial. “What happened deeper than 200 kilometers was just our idea, our models, because obtaining the necessary materials is very difficult.” She notes that there are only a few samples from that depth.
These new samples, obtained from depths ranging from 280 to 470 km below the Earth's surface, provide the first real-world test of theoretical data on mantle chemistry. According to Weiss, one conclusion is that oxidized molten material is found deeper than previously thought. Kimberlites, the eruptive rocks from which diamonds come to the surface, are oxidized, so researchers previously thought they couldn't form much deeper than 300 km. However, these results suggest that oxidized rocks are found deeper, suggesting that kimberlites could, too.
According to Weiss, diamond-forming reactions likely occur when carbonate fluids are pulled downward by tectonic plates, bringing oxygen-rich minerals into contact with mantle metal alloys. (Chemists believe diamonds could also form by precipitation from carbon-rich liquids that cool as they rise through the mantle, similar to how sugar crystallizes from syrup. The new paper doesn't rule out this process.)
RELATED STORIES
—Why do diamonds come in different colors?
Scientists have finally created the elusive meteorite diamond, which is predicted to be 50% harder than terrestrial diamonds.
Research has shown that a 9-mile-thick layer of solid diamonds may be hidden beneath Mercury's surface.
Nickel-rich inclusions may also help explain a strange phenomenon in some diamonds: individual nickel atoms appear to replace carbon in the diamonds' crystal lattice. This was a mystery, says Kopylova, because nickel is much heavier than carbon and shouldn't easily integrate into a crystal structure. “Now, looking at this data, I realize it could simply be a sign of diamond formation at certain depths,” she says. “It would be very interesting to study this further.”
This article originally appeared in Scientific American. © ScientificAmerican.com. All rights reserved. Follow us on TikTok and Instagram, X and Facebook.
TOPICS diamonds

Stephanie Pappas, Social Link Navigator, Live Science Contributor
Stephanie Pappas is a freelance writer for Live Science, covering a wide range of topics, from geological sciences and archaeology to the human brain and behavior. Previously a senior writer for Live Science, she now works as a freelance writer in Denver, Colorado, and regularly contributes to Scientific American and The Monitor, the monthly magazine of the American Psychological Association. Stephanie earned a bachelor's degree in psychology from the University of South Carolina and a graduate certificate in science communication from the University of California, Santa Cruz.
You must verify your public display name before commenting.
Please log out and log back in. You will then be asked to enter a display name.
Exit Read more

We are only just beginning to learn what the Earth's inner core is really made of.
A huge source of the rare earth metal niobium was brought to the surface when the supercontinent broke apart.

Bizarre liquid 'fireworks' occur when scientists try to mix immiscible liquids.

Scientists have discovered that mysterious giant structures on the floor of the North Sea seem to defy our knowledge of geology.

Enormous formations deep beneath the Earth's surface appear to be causing gigantic volcanic eruptions

'Missing' Mercury Meteorites May Finally Be Found on Earth
Latest geology news

Strange glass in Australia appears to be formed by a giant asteroid impact, but scientists 'have yet to find the crater'
A huge source of the rare earth metal niobium was brought to the surface when the supercontinent broke apart.

Narusawa Ice Cave: A lava tube filled with 10-foot-tall ice columns at the base of Mount Fuji.

A giant sand 'slug' is crawling across floodplains in Kazakhstan, but may soon freeze in place.

Scientists have discovered that the geology that supports the Himalayas is not what we thought.

Lugarema: The 'disappearing lake' in Northern Ireland that mysteriously dries up and refills within hours
Latest news

“Gold coins started appearing one after another”: 1,400-year-old hoard of money and jewelry discovered near the Sea of Galilee

Rare wampum beads discovered in 17th-century Newfoundland colony

A 1-million-year-old skull from China holds clues to the origins of Neanderthals, Denisovans, and humans.
NASA launches special mission to study Earth's mysterious “halo”

A mysterious creature discovered in Peru's 'forbidden cloud forest' is a new species of marsupial.

A 95-million-year-old, never-before-seen 'tiny skull' of a crocodile-like creature has been discovered in Montana.
LATEST ARTICLES
NASA launches special mission to study Earth's mysterious “halo”
Live Science magazine is part of Future US Inc., an international media group and leading digital publisher. Visit our corporate website.
- About Us
- Contact Future experts
- Terms and Conditions
- Privacy Policy
- Cookie Policy
- Accessibility Statement
- Advertise with us
- Web notifications
- Career
- Editorial standards
- How to present history to us
© Future US, Inc. Full 7th Floor, 130 West 42nd Street, New York, NY 10036.
var dfp_config = { “site_platform”: “vanilla”, “keywords”: “type-news-daily,type-crosspost,exclude-from-syndication,serversidehawk,videoarticle,van-enable-adviser-
Sourse: www.livescience.com