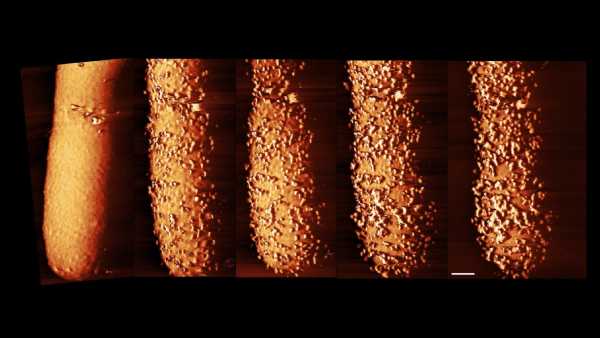
Polymyxins, a class of antibiotics, compel bacteria to manufacture and discard their external “protection,” creating an opening for the antibiotic to then penetrate and eradicate the bacteria.(Image credit: © Imperial College London)
Researchers have unveiled fresh visuals displaying, in remarkable clarity, how antibiotics conquer harmful bacteria by puncturing the microbes’ outer layers and accessing their interior components.
The antibiotics, known as polymyxins, were seen inducing the defensive layers around Escherichia coli bacterial cells to develop protrusions and protuberances. Subsequently, the bacteria cast off these exterior coverings, allowing the antibiotic access into the cells.
You may like
-
How the struggle between viruses and bacteria may aid us in defeating superbugs
-

Alexander Fleming discovers a peculiar mold in his petri dish, leading to the unintentional finding of the first antibiotic — Sept. 28, 1928
-
‘Nested Russian doll’ virus conceals itself within a hazardous fungus, amplifying the threat to humans
Gram-negative bacteria encompass a diverse category of microorganisms characterized by a dual-membrane structure around each cell; these two membranes enclose a cell wall. E. coli, Salmonella, and Shigella — a bacterial strain responsible for dysentery — are illustrative examples of gram-negative bacteria.
Polymyxins offer assistance in managing infections arising from gram-negative bacteria that have built up resistance to other antibiotic treatments; their mechanism involves targeting the outermost of the bacteria’s two membranes, functioning as a shield to bar antibiotics. Nevertheless, the precise manner in which these antibiotics circumvent this defense remains unclear.
“Polymyxins are a critical line of defense against Gram-negative bacteria, which are responsible for numerous life-threatening infections resistant to medication,” stated study co-author Bart Hoogenboom, a biophysicist from UCL, in the statement. “Gaining insight into their mode of action is vital.”
In the recent study, which appeared on Sept. 29 in the journal Nature Microbiology, the research team successfully captured images of the antibiotic at work. Employing a methodology referred to as atomic force microscopy, the scientists navigated a minuscule needle to and fro across the bacteria, creating a visual representation of their configurations. This process enabled them to observe the alterations in the bacteria as the polymyxin initiated its assault.
Polymyxins caused E. coli to rapidly generate minute elevations and projections on its external covering, the team observed. Concurrent with the expansion of these protuberances, the bacteria relinquished its protective layer, engendering voids in that external membrane, facilitating the passage of antibiotics and subsequent cell death.
“Our images of the bacteria provide direct evidence of the extent to which polymyxins can undermine the bacterial shield,” Borrelli clarified. “It resembles a scenario in which the cell is compelled to produce ‘bricks’ for its outer perimeter at an accelerated pace, leading to a disruption in this perimeter, thereby permitting antibiotic penetration.”
RELATED STORIES
—Can we undo antibiotic resistance that renders formerly life-saving medicines obsolete?
—An entirely novel class of antibiotic targets drug-resistant superbugs
—Caffeine potentially aids E. coli in building antibiotic resistance — necessitating further investigation
Critically, polymyxins demonstrate efficacy solely against bacteria undergoing active growth, excluding those transitioning into a dormant state. Bacteria occasionally undergo a dormant phase as a survival strategy in response to harsh environments, persisting for extended durations sans nourishment, growth, or reproduction, only to re-activate upon the amelioration of conditions. In their dormant state, bacteria lack the capacity to expand their external protective shield, thereby impeding the antibiotic’s ability to escalate production akin to that in actively proliferating bacteria.
“Our forthcoming objective is to leverage these discoveries to enhance the potency of antibiotics,” Hoogenboom indicated. “A potential tactic involves integrating polymyxin therapy — paradoxically — alongside interventions that foster shield generation and/or rouse ‘dormant’ bacteria, facilitating the comprehensive removal of these cells.”
Disclaimer
This article is intended solely for informational purposes and is not a replacement for professional medical guidance.

Skyler WareSocial Links NavigationLive Science Contributor
Skyler Ware serves as a freelance science correspondent, specializing in chemistry, biology, paleontology, and Earth science. She participated as a 2023 AAAS Mass Media Science and Engineering Fellow at Science News. Her published works extend to Science News Explores, ZME Science, and Chembites, to name a few. Skyler possesses a Ph.D. in chemistry obtained from Caltech.
Before commenting, you must verify your displayed name.
Sign out and subsequently sign back in to initiate the prompt for your display name entry.
LogoutRead more
How the struggle between viruses and bacteria may aid us in defeating superbugs

Alexander Fleming discovers a peculiar mold in his petri dish, leading to the unintentional finding of the first antibiotic — Sept. 28, 1928
‘Nested Russian doll’ virus conceals itself within a hazardous fungus, amplifying the threat to humans
You may not really be allergic to penicillin. Here’s how to find out if you are.
Mitochondria aren’t only the ‘powerhouses of cells’ — they also battle germs

Mystery of why sea stars keep turning into goo finally solved — and it’s not what scientists thought
Latest in Medicine & Drugs
‘Groundbreaking’ gene therapy is first treatment for Huntington’s disease to slow the condition
Who is eligible for this year’s COVID vaccine? Everything you need to know
CDC committee votes to change measles vaccine guidance for young children

RFK’s handpicked advisers are coming for the childhood vaccine schedule. Here’s what to know.

Breakthrough cystic fibrosis drug that extends life by decades earns its developers a $250,000 ‘American Nobel’
Sourse: www.livescience.com





