MEMBER EXCLUSIVE

mRNA study made the remarkably swift creation of COVID-19 vaccine contenders during the global health crisis feasible. However, in a larger sense, this tech could reshape numerous medical fields beyond just preventative inoculations for communicable diseases.(Image credit: Adrián Astorgano)ShareShare by:
- Copy link
- X
Share this article 6Join the conversationFollow usAdd us as a favored source on GoogleNewsletterSubscribe to our newsletter
On Dec. 31, 2019, initial reports surfaced regarding a perplexing instance of pneumonia of undetermined origin propagating in China. By March 11, 2020, the World Health Organization declared the COVID-19 outbreak as a global pandemic.
On March 16, the premier COVID-19 immunization entered the stage of clinical evaluations.
You may like
-
From gene therapy innovations to preventable disease outbreaks: The health forecasts anticipated for 2026
-
‘This is a completely different level of anti-vaccine engagement than we’ve ever seen before,’ shares epidemiologist Dr. Seth Berkley
-
COVID-19 mRNA shots can incite the immune response to spot and eradicate cancer, investigations uncover
The initial coronavirus immunizations were revolutionary due to their evolution from conception to expansive manufacturing in merely months. However, their uniqueness also stemmed from the use of a novel method for stimulating the body’s defenses — one that underwent intensive assessment for decades in preparation for implementation at this pivotal juncture.
Central to these vaccines was messenger RNA (mRNA), a lesser-known counterpart of DNA. The efficacy of the mRNA design lies in the capacity for exceedingly swift vaccine output after a pathogen’s genetic details are decoded; while traditional vaccine production demands months or years, mRNA counterparts can be produced in only weeks. Consequently, mRNA, once confined to high school biology lessons and specialized zones of biomedical study, abruptly entered mainstream awareness — triggering relentless disinformation and debate thereafter.
While mRNA-centric COVID-19 immunizations represent the most well-known employment of this molecule, global scientists have identified supplementary roles for mRNA tech extending beyond inoculation. Its potential is being examined for pioneering treatments targeting malignancies and autoimmune conditions, together with gene-editing approaches for inherited ailments. Nevertheless, this aspiration may remain unmet within the U.S., where the national administration has proclaimed opposition to this encouraging tech.
This fresh stance clashes with the prior endorsement of mRNA vaccines by the Trump leadership.

Science Spotlight examines developing research in depth, furnishing you, our audience, with the perspectives essential to comprehending these advancements. Our features underscore patterns in varied disciplines, illuminate how cutting-edge studies reshape established concepts, and reveal how the perception of our inhabited world is being transformed through scientific exploration.
“We truly must acknowledge President Trump’s instrumental role in exposing the mRNA paradigm to the world via his direction of Operation Warp Speed,” stated Jeff Coller, the distinguished Bloomberg professor specializing in RNA biology and treatments at Johns Hopkins University. “The president merits a victory lap.” Conversely, the subsequent Trump administration is vigorously dismantling this inheritance, Coller informed Live Science.
Vaccine skeptic Robert F. Kennedy Jr. now leads the Department of Health and Human Services (HHS), and detractors of both standard and mRNA-reliant immunizations hold positions on the nation’s leading advisory board concerning shots. Since Trump’s inauguration, federal researchers have experienced widespread dismissals, stalled funding, and memos cautioning them to disclose participation in research domains targeted by the administration, notably mRNA vaccines.
The COVID-19 immunizations developed by Pfizer-BioNTech and Moderna leverage mRNA as their foundation. Their conception, initial endorsement, and ensuing authorization signaled passage into a novel age of medical practice.
These initiatives immediately stifled mRNA study and advancement inside the U.S., Coller disclosed to Live Science. Subsequently, this past August, HHS terminated close to half a billion dollars allocated for mRNA immunization expansion.
You may like
-
From gene therapy innovations to preventable disease outbreaks: The health forecasts anticipated for 2026
-
‘This is a completely different level of anti-vaccine engagement than we’ve ever seen before,’ shares epidemiologist Dr. Seth Berkley
-
COVID-19 mRNA shots can incite the immune response to spot and eradicate cancer, investigations uncover
“I was genuinely taken aback by this,” conveyed Jordan Green, leading the Biomaterials and Drug Delivery Laboratory at Johns Hopkins, whose team engineers mRNA treatments along with distribution mechanisms designed to channel the molecule into the organism.
These funding reductions are spreading apprehension throughout the biotechnology field, leading stakeholders to deliberate on the reliability of establishing facilities within the U.S., and ponder whether their mRNA investments may benefit from allocation abroad. “It’s regrettable, as it constitutes an avoidable misstep; no rationale underlies it,” Green lamented.
Currently, HHS seems to be primarily retreating from mRNA vaccines; it affirmed that “alternative uses of mRNA technology” would not be susceptible to these cutbacks. Yet, “the industry is skeptical,” Coller communicated with Live Science.
According to Grant Witness, an initiative tracking scientific donations throughout the Trump management, mRNA study unrelated to immunizations has previously faced grant terminations and financial freezes. Consequently, even without explicit targeting, its preservation isn’t assured. The project’s repository denotes that the National Institutes of Health (NIH), an arm of HHS, ended contributions supporting schemes advancing mRNA-oriented therapies addressing cancer, Alzheimer’s, pulmonary arterial hypertension, and HIV, alongside basic research projects assessing mRNA functionality in healthy and pathological cells.
Below is an account of what the United States risks sacrificing if the national government broadly diminishes its stake in mRNA medications, succeeding decades devoted to readying this technology for implementation.
Cancer treatment
At a fundamental level, mRNA bears close resemblance to DNA — and it abounds within human cells. These commonplace “messengers” transcribe commands originating from DNA and convey them to cellular zones — specifically, protein synthesis hubs that create intricate molecules fulfilling most tasks within cells. mRNA also undertakes additional essential cellular tasks, assisting with directing gene activation and its extent.
Throughout decades preceding the pandemic, dedicated scientists intensively studied mRNA, discovering aspects of this molecular class within the body and its potential to remedy illnesses and avert sickness.
“My involvement began around age 21, during the ’90s, when only a handful of global experts understood mRNA,” Coller stated. Discoveries originating from Coller’s laboratory further underpinned the advancement of Spikevax, Moderna’s COVID-19 immunization. Although COVID-19 vaccines remain the most well-known modern utility of mRNA, this molecule’s influence spans beyond solely this.
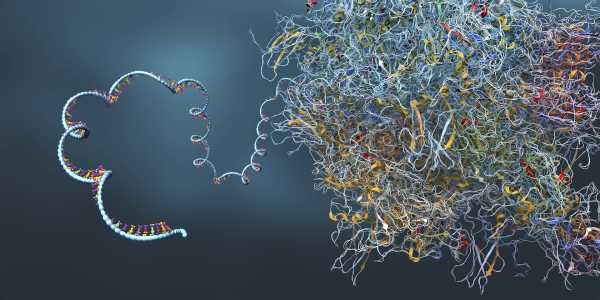
RNA shares a structure resembling DNA, while possessing a single, rather than dual intertwined strands. mRNA serves as a “messenger” conveying details from DNA externally into the cell.
The inaugural therapeutic mRNA corporation was founded during 1997, not targeting contagious ailments but instead centering around cancer interventions. Though its methodology hasn’t succeeded during human tests, alternative approaches employing mRNA-focused cancer treatment have gained momentum.
Cancer vaccinations serve as a leading example, however, within this framework, the designation “vaccine” is “somewhat imprecise,” stated Dr. Vinod Balachandran, a pancreatic cancer surgeon-scientist and director of The Olayan Center for Cancer Vaccines (OCCV) situated at Memorial Sloan Kettering Cancer Center. Unlike preemptive treatments, such as a COVID shot, cancer vaccinations are “administered to patients as a treatment; it embodies therapy,” he mentioned.
These therapeutic interventions mirror conventional vaccines through instilling immune proficiency recognizing antigens, entities acting as “warning indicators” revealing invading elements, contaminants, or damaged cells. Encompassed examples entail SARS-CoV-2’s spike protein — the viral agent sparking COVID-19 — alongside specific cancer cell molecules. Balachandran coupled with his peers focused upon pancreatic cancer, associated with merely a 13% five-year relative survival rate — signifying recent pancreatic cancer diagnoses correlate with approximately 13% odds of prolonged survival of five years relative to the broader population.
These investigators determined that, within infrequent pancreatic cancer survivors, the immune system identifies and suppresses tumor recurrence. The collaborative group aimed to replicate similar immunological comprehension through assessing tumor genetics to expose unique antigens they manifest. Subsequently, they derive tailored vaccines targeting such molecules.
“We believed at the time [on initiating our undertaking] — back during 2017 — that optimal technology for rapid, patient-centered cancer immunization necessitated RNA usage,” Balachandran disclosed. Upon pinpointing a patient’s specific cancer genetics, a bespoke mRNA vaccine targeting multiple antigens may be conceived spanning merely weeks. Conversely, traditional vaccinations, requiring antigens to propagate within laboratories while undergoing purification, would demand multiple months for production.
“Speed constitutes an indispensable component of cancer vaccination,” Balachandran commented. “These patients confront lethal malignancies necessitating prompt therapy. Consequently, protracted delays aren’t viable.”
Thus far, the team has noted gains. Within an initial investigation, they addressed 16 patients having experienced surgery due to pancreatic ductal adenocarcinoma (PDAC), prevalent among pancreatic malignancies, correlating with 10% to 12% prospects of a five-year survival, yet faring more favorably through surgical cancer elimination.
Balachandran cited, nine weeks following the procedure, each patient’s tumor sample underwent assessments while establishing a tailored vaccine accompanied by subsequent cancer therapies, like chemotherapy. Half the patient population exhibited favorable vaccine reactions, yielding immunological entities enduring for nearing four years, projected spanning seven years on average, “including some lasting beyond a decade,” Balachandran cited.
Vaccine respondents demonstrated conspicuously mitigated recurrence hazards throughout the succeeding three years relative to non-responders, encompassing six displaying no such traces transpiring within this temporal space. An intermediate examination now appraises the vaccine spanning 260 participants, measuring the efficiency of hindering/averting recurrence relative to standard interventions.
“Efforts aimed at augmenting outcomes among these gravely afflicted patients will transform their individual experiences, encompassing familial well-being — connoting significant benefits,” Balachandran noted. “Substantially, alternative immunotherapies combined alongside rival remedies have proven primarily futile.”
Initially, producing vaccines exceeded two months during trial instances, partially resulting from the sample consignment targeting overseas associates located within BioNTech. “Future processes are confidently envisioned as highly accelerated,” possibly spanning a month, Balachandran remarked.
Other scientists develop off-the-shelf vaccines to potentially mitigate interim circumstances while patients await customized vaccinations. These involve merging mRNA molecules inciting standardized, preparatory immunological defenses targeting malignancies. During tests administering mice comprising various solid tumor categories, scientists directed through Dr. Elias Sayour, a pediatric oncologist based at the University of Florida, indicated such immunization sparks standalone anticancer immunological response. Furthermore, implemented alongside differing oncological treatment practices, integrating mRNA may augment the efficacy emanating from concurrent treatment courses, Sayour’s team discovered.

A researcher working on a lung cancer vaccine in the O
