“`html
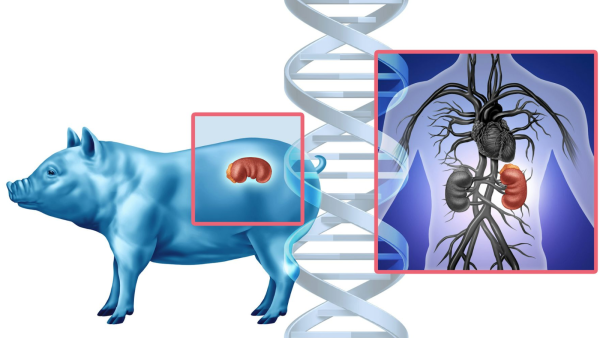
Although investigations into human-pig chimeras are on hold indefinitely, xenotransplantation is progressing. (Image credit: wildpixel/iStock via Getty Images Plus)ShareShare by:
- Copy link
- X
Share this article 0Join the conversationFollow usAdd us as a preferred source on GoogleNewsletterSubscribe to our newsletter
In a surgical room in New York on a certain day in October 2025, doctors achieved a milestone in medicine by grafting a genetically altered pig’s kidney into a living person as part of a trial. The kidney was crafted to resemble human tissue and cultivated within a pig, serving as a substitute for awaiting a human organ provider who might never materialize. For many years, this concept resided on the fringes of speculative science. Now, it’s an actuality.
The individual is among six participating in the primary clinical assessment of pig-to-human kidney implants. The purpose: to ascertain if genetically modified pig kidneys can effectively take the place of failing human kidneys.
A decade prior, researchers were pursuing a distinct strategy. Instead of modifying the genes of pigs to render their organs compatible with humans, they endeavored to cultivate human organs — composed solely of human cells — within pigs. However, in 2015, the National Institutes of Health suspended financial support for that initiative to examine its moral implications. The suspension is still in effect.
You may like
-

Scientists convert a kidney from blood type A to universal type O and implant it in a brain-dead recipient
-
Restrictions on fetal tissue research would threaten progress on breakthrough treatments for devastating diseases — and yet not prevent a single abortion
-

Neanderthals could be brought back within 20 years — but is it a good idea?
As someone specializing in bioethics and philosophy, who has devoted considerable time studying the morality of employing organs grown inside animals — including contribution to a federally funded working group examining oversight for research on human-animal chimeras — I was puzzled by the verdict. The prohibition assumed the risk involved making pigs excessively human. Yet, regulators now appear at ease making humans somewhat more piglike.
Why does placing pig organs into humans deemed ethical, while cultivating human organs inside pigs is not?
Urgent need fuels xenotransplantation
It is easy to disregard the critical situation prompting these trials. Over 100,000 individuals in America are awaiting organ donations. The need surpasses the provision, and countless individuals pass away annually before one becomes obtainable.
For decades, scientists have explored across different species for assistance — from primate hearts in the 1960s to genetically altered pigs presently. The hurdle has consistently been the defense mechanism of the immune system. The body deems cells it doesn’t identify as its own as invaders. Consequently, it eliminates them.
A recent instance highlights this delicacy. A man residing in New Hampshire was given a gene-edited pig kidney in January 2025. Nine months later, it required removal owing to diminishing function. While this limited triumph provided researchers optimism, it also served as a reminder that rejection continues to be a primary concern when transplanting organs between species, otherwise referred to as xenotransplantation.
First clinical trial of pig kidney transplants is underway – YouTube

Watch On
Scientists are endeavoring to overcome transplant rejection through generating an organ that the human system might accept, incorporating a handful of human genes and eradicating specific pig genes. However, those receiving these gene-altered pig organs necessitate potent medications to subdue the immune system, both during and for a duration after the transplant process; even with this, rejection might not be prevented. Even transplants between humans demand lifelong immunosuppressants.
This is why an alternative method — cultivating organs from a patient’s personal cells — appeared encouraging. This involved impairing the genes responsible for pig embryos forming a kidney, then inserting human stem cells within the embryo to occupy the void where a kidney would typically be. Consequently, the pig embryo would develop a kidney genetically identical to a prospective patient, theoretically mitigating the prospect of rejection.
You may like
-

Scientists convert a kidney from blood type A to universal type O and implant it in a brain-dead recipient
-
Restrictions on fetal tissue research would threaten progress on breakthrough treatments for devastating diseases — and yet not prevent a single abortion
-

Neanderthals could be brought back within 20 years — but is it a good idea?
Despite being straightforward in theory, the implementation is technically intricate due to human and pig cells advancing at differing rates. Regardless, five years before the NIH prohibition, researchers had already achieved something analogous, cultivating a mouse pancreas inside a rat.
Cross-species organ development wasn’t just wishful thinking — it was a validated proof of principle.
Ethics of originating organs in alternate species
The anxieties motivating the NIH prohibition in 2015 on inserting human stem cells into animal embryos didn’t arise from apprehensions about scientific setbacks, but instead from moral quandaries.
Decision-makers feared the human cells might spread throughout the animal’s system — potentially even the brain — thereby obscuring the distinction between human and animal. The NIH cautioned about probable “alterations to the animal’s cognitive state.” The Animal Legal Defense Fund, an organization advocating for animal welfare, contended that should such chimeras attain human-like consciousness, they ought to be regarded as human research participants.
The concern focuses on the prospect that an animal’s ethical position — specifically, the extent to which an entity’s interests are morally significant and the degree of safeguarding it warrants – might evolve. A superior ethical position mandates enhanced care, because it carries vulnerability to greater forms of detriment.
Consider the harm inflicted by disturbing a sentient animal in contrast to the harm caused by disturbing an animal possessing self-awareness. A sentient animal — meaning one adept at sensing sensations like pain or pleasure — would detect the pain and strive to evade it. Conversely, an animal that’s self-aware — that is, one possessing the ability to reflect on having those experiences — would not only sense the pain, but understand that it is itself the subject of said pain. This latter form of detriment is more profound, encompassing not just sensation but also understanding.
Hence, the NIH’s worry is that if human cells migrate into an animal’s brain, they might introduce novel forms of experience and suffering, thereby elevating its ethical position.

How much humanity must pigs possess before being considered a part of the human race? The flawed logic behind the NIH prohibition
Nevertheless, the rationale underpinning the NIH’s prohibition is defective. Should particular cognitive abilities, for example, self-awareness, bestow a superior moral position, then it would follow that regulators would express comparable concern about introducing dolphin or primate cells into pigs, as they do when introducing human cells. However, this is not the case.
In practice, the moral boundary of beings whose interests hold significance is drawn not around self-awareness but around species membership. Regulators safeguard all humans from damaging research owing to their humanity, not due to particular cognitive capacities, such as the aptitude to experience pain, employ language, or engage in abstract reasoning. Actually, a significant number of individuals lack such capacities. Moral consideration stems from this association, not from possessing a specific form of understanding. No research objective can warrant violating the most essential interests of human beings.
If a pig embryo infused with human cells genuinely evolved into something close enough to be considered a member of humanity, then current research guidelines would dictate it’s owed human-level consideration. Yet the mere existence of human cells does not transform pigs into humans.
RELATED STORIES
—First-ever pig-to-human lung transplant attempted in brain-dead person in China
—In a 1st, scientists grow human kidneys inside developing pig embryos
—’We have combined two marvels of modern medicine’: Woman gets pig kidney and heart pump in groundbreaking procedures
The pigs engineered for kidney implants already harbor human genes, yet they are not referred to as half-human beings. When an individual donates a kidney, the recipient doesn’t become a member of the donor’s family. Yet current research policies regard a pig possessing a human kidney as though it might.
There may exist valid justifications for objecting to employing animals as living organ factories, encompassing welfare concerns. However, the underlying reason behind the NIH ban, asserting that human cells could render pigs excessively human, rests on a misunderstanding of what confers moral status to beings — and humans specifically.
This revised article is republished from The Conversation under a Creative Commons license. Read the original article.

Monika PiotrowskaAssociate Professor of Philosophy, University at Albany, State University of New York
A philosopher and bioethicist whose investigations center around theoretical and ethical quandaries stemming from advancements in genetics and biotechnology.
Show More Comments
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
LogoutRead more

Scientists convert a kidney from blood type A to universal type O and implant it in a brain-dead recipient
Restrictions on fetal tissue research would threaten progress on breakthrough treatments for devastating diseases — and yet not prevent a single abortion

Neanderthals could be brought back within 20 years — but is it a good idea?
From gene therapy breakthroughs to preventable disease outbreaks: The health trends that will shape 2026
