
mRNA exploration facilitated the remarkably quick generation of COVID-19 vaccine prospects during the global health crisis. However, more comprehensively, this technology has the capacity to transform numerous sectors of healthcare beyond disease-averting vaccines for contagious ailments.(Image credit: Adrián Astorgano)
On Dec. 31, 2019, the primary accounts surfaced concerning an enigmatic pneumonia with an unidentified etiology spreading in China. On March 11, 2020, the World Health Organization designated COVID-19 as a global pandemic.
On March 16, the introductory COVID-19 immunization commenced clinical testing.
You may like
-

Breaking Down RFK Jr.’s (Numerous) Inaccurate Assertions Regarding COVID Vaccinations
-
‘This signifies a totally distinct echelon of anti-vaccine participation compared to what we’ve previously witnessed,’ conveys epidemiologist Dr. Seth Berkley
-
COVID-19 mRNA immunizations are capable of provoking the immunological apparatus to identify and eliminate malignancy, according to research
The initial coronavirus vaccines constituted a transition in thinking because they progressed from conception to extensive manufacturing within a matter of months. Nevertheless, they were additionally exceptional considering they exploited an innovative avenue to incite the immune response — one that had undergone exhaustive study for decades to ensure preparedness for deployment during this pivotal juncture.
The solution to these vaccines was messenger RNA (mRNA), the lesser-known counterpart of DNA. The strength of the mRNA framework resides in the reality that immunizations can be generated remarkably swiftly once a pathogen’s genetic makeup has been examined; conventional vaccine production necessitates months or years, whereas mRNA vaccines can be fabricated in mere weeks. Accordingly, despite once being confined to high school biology lectures and specialized areas of biomedical research, mRNA was unexpectedly propelled into the public consciousness — and once it arrived, it gave rise to persistent disinformation and controversy.
While mRNA-centered COVID-19 vaccines represent the most renowned implementation of the molecule, investigators worldwide have identified supplementary uses for mRNA technology extending beyond the field of immunizations. They are probing its application for groundbreaking remedies for malignancy and autoimmune ailments, as well as gene-altering interventions for genetic conditions. Nevertheless, this potential might remain unrealized in the United States, where the federal administration has proclaimed a war against this encouraging technology.
This contemporary stance contradicts the prior embrace of mRNA vaccines by the Trump administration.

Science Spotlight provides a more in-depth investigation of emerging science and furnishes you, our readership, with the understanding you necessitate concerning these progressions. Our articles emphasize patterns in diverse domains, how novel research is reshaping established concepts, and how the depiction of the world we inhabit is undergoing alteration thanks to science.
“We truly must acknowledge President Trump for acquainting the global population with the mRNA platform through his guidance in Operation Warp Speed,” remarked Jeff Coller, the Bloomberg distinguished professor of RNA biology and therapeutics at Johns Hopkins University. “The president ought to be celebrating his triumph.” Yet rather, the second Trump administration is actively eroding this heritage, Coller conveyed to Live Science.
Vaccine skeptic Robert F. Kennedy Jr. currently presides over the Department of Health and Human Services (HHS), and adversaries of both conventional and mRNA-centered immunizations hold positions on the nation’s most authoritative vaccine advisory panel. Subsequent to Trump’s inauguration, federal researchers have encountered extensive dismissals, financial impasses, and memorandums cautioning them to disclose their involvement in research areas that the administration has aimed at, including mRNA vaccines.
The COVID-19 vaccines produced by Pfizer-BioNTech and Moderna employ mRNA as their foundation. Their creation, initial authorization, and subsequent endorsement signified a transition into a novel epoch of medicine.
These measures exerted an immediate inhibiting impact on mRNA exploration and advancement in the U.S., Coller informed Live Science. And then, in August, HHS terminated nearly half a billion dollars’ worth of investment into mRNA vaccine development.
You may like
-

Breaking Down RFK Jr.’s (Numerous) Inaccurate Assertions Regarding COVID Vaccinations
-
‘This signifies a totally distinct echelon of anti-vaccine participation compared to what we’ve previously witnessed,’ conveys epidemiologist Dr. Seth Berkley
-
COVID-19 mRNA immunizations are capable of provoking the immunological apparatus to identify and eliminate malignancy, according to research
“Frankly, I was shocked to witness this,” conveyed Jordan Green, chief of the Biomaterials and Drug Delivery Laboratory at Johns Hopkins, whose laboratory is formulating both mRNA therapies and mechanisms for transporting the molecule into the physique.
These cutbacks are disseminating apprehension throughout biotech, prompting stakeholders to contemplate whether it’s secure to establish operations in the U.S., or whether their mRNA investments would be more advantageously deployed abroad. “It’s just regrettable because it’s an avoidable blunder; there exists no rationale,” Green expressed.
At present, HHS appears to be primarily retreating from mRNA vaccines; it observed that “other applications of mRNA technology” would remain unaffected by the curtailments. However, “the sector harbors skepticism regarding that,” Coller conveyed to Live Science.
According to Grant Witness, a project tracking scientific subsidies under the Trump administration, mRNA exploration unrelated to immunizations has already been impacted by grant terminations and funding restrictions. Consequently, even in the absence of explicit targeting, it isn’t necessarily being protected. The project’s database demonstrates that the National Institutes of Health (NIH), an affiliate of HHS, terminated grants for projects formulating mRNA-based treatments for cancer, Alzheimer’s, pulmonary arterial hypertension, and HIV, in addition to grants for fundamental exploration regarding how mRNA operates in healthy and diseased cells.
Here’s what the United States risks forfeiting if the federal government generally diminishes its involvement in mRNA medicines subsequent to dedicating decades to readying the technology for its prime.
Cancer treatment
At a molecular level, mRNA constitutes a close relative of DNA — and human cells are saturated with it. These ubiquitous “messengers” duplicate directives from DNA and transmit them to other locations within the cell — notably, to protein-construction zones where the intricate molecules responsible for executing the majority of cellular tasks are produced. mRNA likewise carries out other pivotal functions within cells, such as aiding in regulating which genes are activated and to what extent.
For decades prior to the pandemic, committed researchers scrutinized mRNA, acquiring insights into how this category of molecules operates within the organism and how it might be employed to alleviate ailment and defend against illness.
“I initiated this endeavor when I was approximately 21, during the ’90s, back when approximately 10 individuals globally comprehended the essence of mRNA,” Coller recollected. Findings from Coller’s laboratory subsequently contributed to the formulation of Spikevax, the COVID-19 vaccine manufactured by Moderna. Although COVID-19 vaccines represent the most recognized implementation of mRNA to date, they are by no means the inaugural.
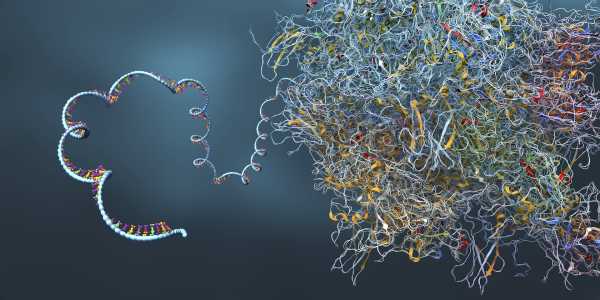
RNA possesses a configuration akin to that of its counterpart DNA, albeit it is composed of solely a singular strand, rather than a dual helix. mRNA functions as a “messenger” that transmits information from DNA out into the cellular milieu.
The pioneering mRNA therapeutics enterprise was inaugurated in 1997, and as opposed to targeting communicable ailments, its focal point centered on cancer treatment. Its methodology has ultimately not yielded success in human trials, but in the meantime, alternative approaches to mRNA-centered cancer treatment have garnered prominence.
Cancer vaccines represent a prominent instance, albeit within this context, the term “vaccine” constitutes “a somewhat imprecise designation,” according to Dr. Vinod Balachandran, a pancreatic cancer surgeon-scientist and director of The Olayan Center for Cancer Vaccines (OCCV) at Memorial Sloan Kettering Cancer Center. Rather than being administered preventatively, akin to a COVID vaccine, cancer vaccines are “administered to patients as a therapeutic intervention; it is a therapy,” he clarified.
These therapies bear resemblance to conventional vaccines in that they educate the immune system to identify antigens, which are substances functioning as “red flags” for extraneous invaders, toxins, or diseased cells. These encompass, for instance, the spike protein of SARS-CoV-2 — the virus responsible for COVID-19 — and specific molecules present on cancer cells. Balachandran and his colleagues have concentrated on pancreatic cancer, which exhibits a five-year relative survival rate of merely 13% — implying that individuals newly diagnosed with pancreatic cancer possess approximately 13% the likelihood of surviving the ensuing five years in comparison to the general population.
The investigators have ascertained that, within the uncommon cohort of long-term pancreatic cancer survivors, the immune system possesses the aptitude to recognize the cancer and counteract its recurrence. The team aspired to recreate this immunological recognition in other patients by scrutinizing the genetic characteristics of their tumors to discern the distinct antigens they express. Subsequently, they formulate tailored vaccines that target these molecules.
“We perceived at that juncture [upon embarking on this endeavor] — this dates back to 2017 — that the optimal technology for expedited custom cancer vaccination for patients entailed the utilization of RNA,” Balachandran recounted. Once the specific cancer genetics of a given patient are known, a personalized mRNA vaccine targeting multiple antigens can be fashioned within a matter of weeks. Conventional vaccines necessitating the cultivation and purification of antigens within the laboratory would necessitate numerous months for production.
“For cancer vaccination, rapidity assumes paramount importance,” Balachandran emphasized. “These are patients confronting lethal malignancies, necessitating rapid treatment. Consequently, we lack the advantage of protracted delays.”
Thus far, the team has observed a modicum of success. Within an early-phase trial, they administered treatment to 16 patients who had undergone surgery for pancreatic ductal adenocarcinoma (PDAC), the most prevalent manifestation of pancreatic cancer, which exhibits a five-year survival rate of approximately 10% to 12%, although their prognosis improves modestly when their cancer is amenable to surgical removal.
Within nine weeks post-surgery, Balachandran reported, each patient’s tumor tissue underwent analysis, and a personalized vaccine was fashioned and administered alongside post-operative cancer therapies, such as chemotherapy. Half of the patients exhibited a response to their vaccine, generating immune cells that have endured for nearly four years to date, and estimations propose that they could persist for an average of seven years, “with some persisting even beyond a decade,” Balachandran conveyed.
The vaccine responders displayed a notably diminished risk of recurrence throughout the ensuing three years compared to those who did not respond, with six exhibiting no indications of recurrence during that timeframe. A mid-stage trial is currently assessing the vaccine in approximately 260 individuals to ascertain its efficacy in delaying or averting recurrence in comparison to standard treatment.
“Anything we can undertake to ameliorate outcomes for these patients who are genuinely in need of assistance, I believe, will be transformative for the field, for them, for their families — it will hold substantial significance,” Balachandran stated. “All other immune therapies and all other therapies have largely proven unsuccessful.”
Within this preliminary trial, vaccine production spanned over two months, attributable in part to the necessity of transporting samples to overseas collaborators at BioNTech. “We harbor confidence that this can transpire considerably more rapidly” in the future, possibly within a month, Balachandran asserted.
Additional scientists are actively engaged in developing off-the-shelf cancer vaccines capable of bridging the interim until a patient obtains a personalized one. These vaccines employ a blend of mRNA molecules to elicit a generic, first-line immune defense against cancer. Within mice exhibiting diverse types of solid tumors, researchers headed by Dr. Elias Sayour, a pediatric oncologist at the University of Florida, demonstrated that such a vaccine instigates anti-cancer responses independently. Furthermore, when employed in conjunction with another cancer treatment, the mRNA blend can augment the impacts of that therapy, Sayour’s team has determined.

A researcher operating on a lung cancer vaccine within the Ose Immunotherapeutics laboratory situated in Nantes, France.
That team has currently transitioned to human patients, employing a dual-pronged strategy: an off-the-shelf cancer vaccine, succeeded by a personalized counterpart. They have additionally executed trials involving personalized vaccines for the lethal brain cancer glioblastoma, discovering that the injections cultivate a robust, targeted immune response against these tumors, which are typically challenging for the immune system to “see.” Concurrently, at another laboratory, scientists are assessing a personalized vaccine in late-stage trials for the skin cancer
Sourse: www.livescience.com





